FDA 3664 2011-2025 free printable template
Show details
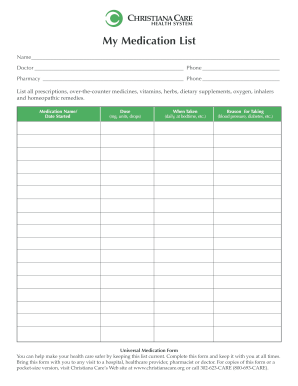
X 800 555-1212 www. fda.gov/Drugs/ResourcesForYou/ucm079489. htm FORM FDA 3664 3/11 Page 1 of 4 888 INFO-FDA These are my medicines as of Enter date as mm/dd/yyyy PSC Publishing Services 301 443-6740 EF My Personal Contacts Allergic Reaction or Other Problem I ve Had With any medicine dietary supplement food skin cleaner medical tape Describe in space below. Be an Active Member of Your Health Care Team DEPARTMENT OF HEALTH AND HUMAN SERVICES Food and Drug Administration My Medicine Record...
pdfFiller is not affiliated with any government organization
Get, Create, Make and Sign printable medication administration record pdf form

Edit your fda templates form online
Type text, complete fillable fields, insert images, highlight or blackout data for discretion, add comments, and more.

Add your legally-binding signature
Draw or type your signature, upload a signature image, or capture it with your digital camera.

Share your form instantly
Email, fax, or share your fda form form via URL. You can also download, print, or export forms to your preferred cloud storage service.
How to edit fda paper online
In order to make advantage of the professional PDF editor, follow these steps:
1
Log in to account. Start Free Trial and sign up a profile if you don't have one yet.
2
Upload a document. Select Add New on your Dashboard and transfer a file into the system in one of the following ways: by uploading it from your device or importing from the cloud, web, or internal mail. Then, click Start editing.
3
Edit fda application form pdf. Rearrange and rotate pages, add and edit text, and use additional tools. To save changes and return to your Dashboard, click Done. The Documents tab allows you to merge, divide, lock, or unlock files.
4
Save your file. Select it from your list of records. Then, move your cursor to the right toolbar and choose one of the exporting options. You can save it in multiple formats, download it as a PDF, send it by email, or store it in the cloud, among other things.
With pdfFiller, it's always easy to work with documents.
Uncompromising security for your PDF editing and eSignature needs
Your private information is safe with pdfFiller. We employ end-to-end encryption, secure cloud storage, and advanced access control to protect your documents and maintain regulatory compliance.
How to fill out debarment certification fda template form

How to fill out FDA 3664
01
Obtain FDA Form 3664 from the FDA website or your local FDA office.
02
Provide the name and address of the firm submitting the application in the designated fields.
03
Clearly state the type of application being submitted (e.g., drug, device, etc.).
04
Fill in the relevant sections about the product’s details including its intended use, formulation, and any associated claims.
05
Complete the certification sections, ensuring all required signatures are obtained.
06
Review the form for accuracy and completeness before submission.
07
Submit the completed form as instructed, either electronically or by mail.
Who needs FDA 3664?
01
Individuals or firms submitting applications for FDA approval for medical devices, drugs, or related products.
02
Manufacturers who are seeking to demonstrate compliance with FDA regulations.
03
Legal representatives or agents acting on behalf of an applicant for FDA approvals.
Fill
form
: Try Risk Free






People Also Ask about
What is the purpose of the FDA Form 3455?
applicants may submit a single FORM FDA 3455, with attachments clearly identifying all clinical investigators with information to disclose and, for each investigator, identifying the study, the specific details of their financial interests and arrangements and the steps taken to minimize the potential for bias.
What is FDA Form 2252?
Form FDA 2252 for submission of annual reports for ANDAs, BLAs, and NDAs; and. Form FDA 2253 for submission of advertising and promotional materials to the Office of Prescription Drug Promotion (OPDP).
What is the purpose of FDA Form 3454?
Form FDA 3454, or the Financial Certification or Disclosure Statement, is used to submit information regarding clinical investigators who participated in the clinical studies. If no clinical studies were performed, simply state: “no clinical studies were performed to test this device.”
What is a Form 1572 per the FDA?
A form that must be filed by an investigator running a clinical trial to study a new drug or agent. The investigator agrees to follow the U.S. Food and Drug Administration (FDA) Code of Federal Regulations for the clinical trial.
What is the FDA Form 3454?
Form FDA 3454, or the Financial Certification or Disclosure Statement, is used to submit information regarding clinical investigators who participated in the clinical studies. If no clinical studies were performed, simply state: “no clinical studies were performed to test this device.”
What is FDA 3455 form?
Certification: Financial Interests and Arrangements of Clinical Investigators. Form FDA 3455. Disclosure: Financial Interests and Arrangements of Clinical Investigators.
For pdfFiller’s FAQs
Below is a list of the most common customer questions. If you can’t find an answer to your question, please don’t hesitate to reach out to us.
How do I modify my FDA 3664 in Gmail?
You can use pdfFiller’s add-on for Gmail in order to modify, fill out, and eSign your FDA 3664 along with other documents right in your inbox. Find pdfFiller for Gmail in Google Workspace Marketplace. Use time you spend on handling your documents and eSignatures for more important things.
How do I complete FDA 3664 online?
Completing and signing FDA 3664 online is easy with pdfFiller. It enables you to edit original PDF content, highlight, blackout, erase and type text anywhere on a page, legally eSign your form, and much more. Create your free account and manage professional documents on the web.
Can I create an eSignature for the FDA 3664 in Gmail?
You may quickly make your eSignature using pdfFiller and then eSign your FDA 3664 right from your mailbox using pdfFiller's Gmail add-on. Please keep in mind that in order to preserve your signatures and signed papers, you must first create an account.
What is FDA 3664?
FDA 3664 is a form used for the reporting of adverse event experiences associated with drugs and devices. It is primarily used by healthcare professionals and manufacturers to communicate safety information to the FDA.
Who is required to file FDA 3664?
Healthcare professionals, manufacturers, and any entity that has knowledge of an adverse event related to a drug or device are required to file FDA 3664.
How to fill out FDA 3664?
To fill out FDA 3664, provide detailed information about the adverse event, including patient demographics, product information, description of the event, and any relevant medical history. Follow the instructions provided on the form carefully.
What is the purpose of FDA 3664?
The purpose of FDA 3664 is to ensure the safety of drugs and medical devices by allowing the FDA to collect and analyze data on adverse events that occur post-marketing.
What information must be reported on FDA 3664?
Information that must be reported on FDA 3664 includes the identity of the product, details of the adverse event, patient information, date of the event, and any actions taken in response to the event.
Fill out your FDA 3664 online with pdfFiller!
pdfFiller is an end-to-end solution for managing, creating, and editing documents and forms in the cloud. Save time and hassle by preparing your tax forms online.

FDA 3664 is not the form you're looking for?Search for another form here.
Relevant keywords
Related Forms
If you believe that this page should be taken down, please follow our DMCA take down process
here
.
This form may include fields for payment information. Data entered in these fields is not covered by PCI DSS compliance.